Glucagon-like Peptide-1 (GLP-1)
The intestinal hormone GLP-1 is a potent incretin, i.e., it stimulates glucose-induced insulin release, and it inhibits gastric emptying. Also, strong evidence links GLP-1 to the control of eating. Endogenous intestinal GLP-1 is implicated in meal termination (= satiation) because (1) the presence of nutrients in the small intestine stimulates GLP-1 release, (2) exogenous GLP-1 inhibits eating primarily by reducing meal size, and (3) IP injection of the GLP-1R antagonist exendin-9 (Ex-9) stimulated eating under some conditions.
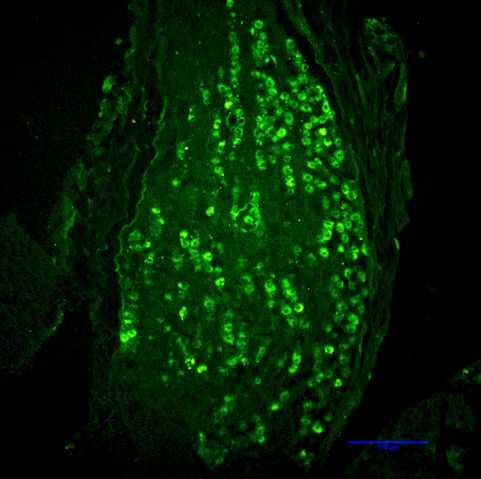
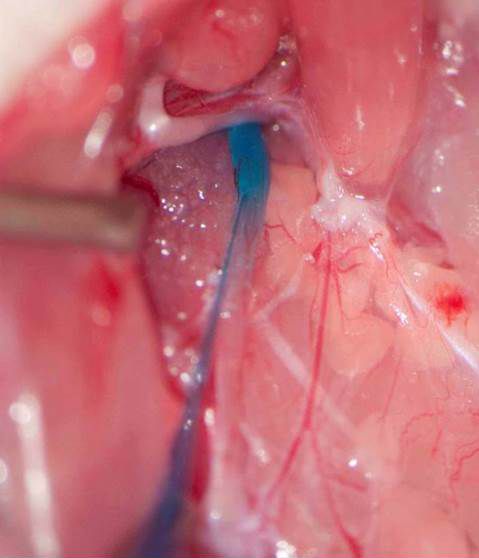
Rapid degradation by dipeptidyl peptidase-IV (DPP-IV) limits GLP-1’s potential as an endocrine signal. Our recent studies indicate, however, that endogenous GLP-1 inhibits eating mainly through a paracrine effect on vagal afferents terminating in the wall of the small intestine. We are currently using different approaches to critically examine this hypothesis. One novel technique is the down-regulation of GLP-1 receptors in abdominal vagal afferents through RNA interference. This is accomplished by bilateral microinjections of a lentiviral vector with a short hairpin RNA into the nodose ganglia, which contain the cell bodies of vagal afferents (Figures 1 and 2).
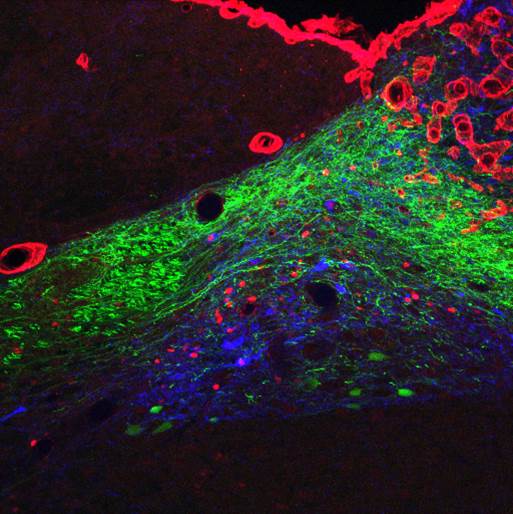
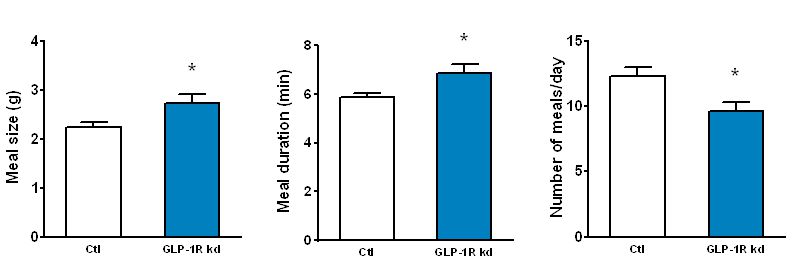
The GLP-1 receptor knockdown leads to permanent increases in meal size and meal duration (Figure 3). Interestingly, GLP-1 also improves glycemic control partly through this mechanism. In addition, we are trying to map the brain areas that are activated by peripheral GLP-1 to identify the neurochemical mechanisms mediating the effects of GLP-1 (Figure 4). Also, we started to unravel the interactions of the peripheral and central GLP-1 systems. All these questions are important for the development of GLP-1-based therapeutic approaches to fight obesity and type-2-diabetes (T2D) and for the putative role of GLP-1 in the obesity- and T2D-curbing effects of gastric bypass surgery.