Gut-brain Communication
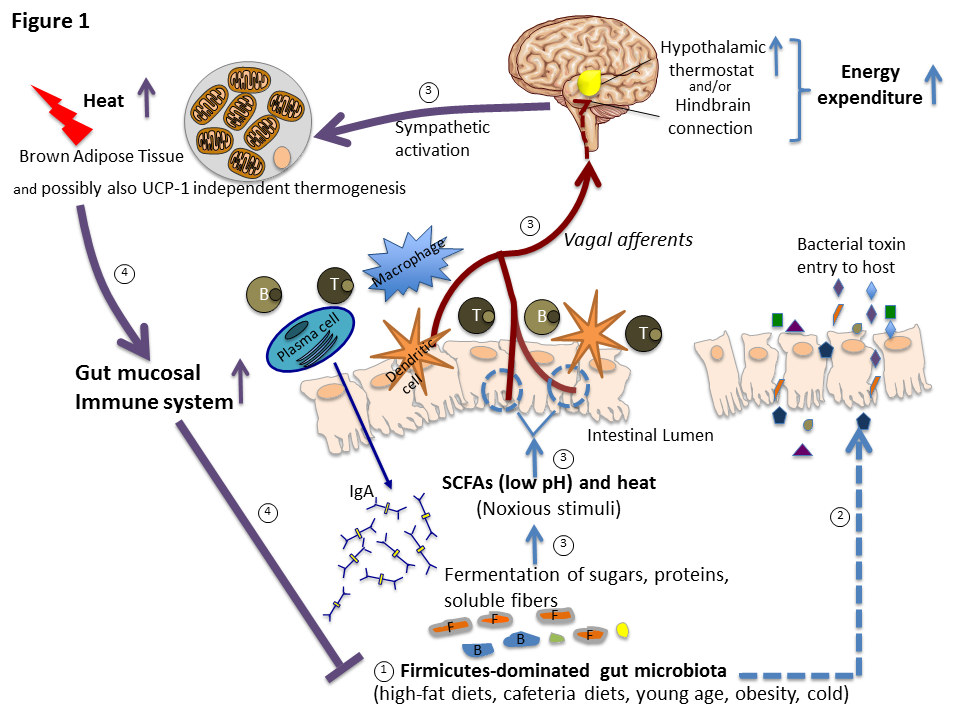
Diet-induced thermogenesis (DIT), i.e., the increase in energy expenditure above/beyond the obligatory stimulation related to the digestion, absorption and metabolism of the ingested nutrients, has often been proposed to be a compensatory mechanism designed to counteract the obesogenic effect of overeating, but no empirical proof of its effectiveness in limiting body weight gain in humans is available. Based on several studies, we recently proposed an immune explanation of DIT, i.e., that it results from the coevolution of host and gut microbiota (especially Firmicutes) that ferment ingested food and proliferate, causing periodic, vagally-mediated increases in thermogenesis aimed at curtailing their expansion (Figure 1, Liao et al., Cell Metabol 23, 971-979, 2016). Because of this evolutionary adaptive significance related to the immune system, DIT is not effective as an “adaptation” to maintain a certain body mass. An increasing population of Firmicutes can be detected by vagal afferent TRPV-1 receptors by a lower pH and an increasing temperature in the gut, a consequence of active metabolic processes of Firmicutes bacteria. Although these vagal afferent TRPV-1 receptors appear so important from an evolutionary perspective for protecting the host’s metabolic health by preventing commensal gut bacteria invasion, their role in activating DIT and in improving gut immunity in DIO has never been critically examined. We plan to uncover their role by employing our sophisticated techniques to interfere with gut-to-brain signaling
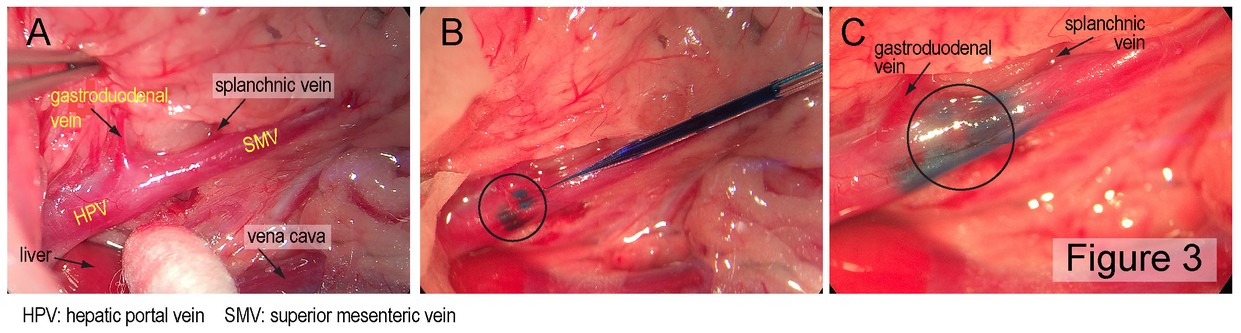
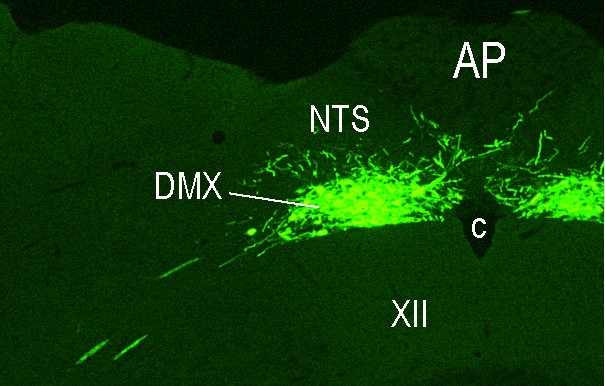
Digestive tract metabolic sensing: A complex system that registers the chemosensory properties of ingested food as well as its amount and macronutrient composition ensures that the body is optimally prepared to digest, absorb and process the ingested food. The various components of this system and how they interact and which brain areas they involve are scarcely known. To take a comprehensive approach to adress these questions, we have teamed-up with Professor Alan Watts (University of Southern California, Los Angeles, CA) and Professor Alan Spector (Florida State University, Tallahassee, FL) to 1) determine the brain targets of nutrient-related sensory information from the mouth, the small intestine, and the hepatic portal vein (HPV), to 2) analyze where in the brain and how the pertinent information is processed and 3) how it triggers the appropriate endocrine and metabolic responses. All three anatomical sites (mouth, small intestine, HPV) contain various sensory elements that transduce nutrient and endocrine information into neural signals. These are transmitted by the cranial nerves including the vagus and glossopharyngeal nerves (from the mouth) and by the vagus and the spinal cord (from the small intestine and the HPV) to the hindbrain. Here they engage more widely distributed brain networks that control eating behavior and metabolic homeostasis. But the precise brain loci receiving this sensory information and whether this cranial/vagal/spinal information segregates in the brain are largely unknown. We will therefore compare and contrast the organization of those hindbrain and forebrain networks that receive sensory information from the mouth, the small intestine and the HPV by these pathways by mapping the precise locations of neurons in the brain that are labeled after injections of an anterogradely transported neurotropic Herpes simplex virus (HSV1-H129) into taste buds, into the submucosa of the small intestine and into the perivascular space of the HPV. Preliminary experiments employing injections into the wall of the HPV have already been performed on the occasion of Professor Watt’s sabbatical in my laboratory last year (see Figures 2 and 3). In future experiments, appropriate nerve lesions will be used to direct the virus injected into the small intestine or HPV along the vagal or the spinal trajectories. To determine where and how the sensory information is connected to the efferent output that controls insulin release from the endocrine pancreas, a retrogradely transported neurotropic Pseudorabies virus (PRV) will be injected into the pancreas together with the anterogradely transported HSV1-H129 injected into each of the three sites (taste buds, wall of the small intestine, wall of the HPV). In addition, we will assess endocrine and metabolic responses to the administration of various metabolites into the oral cavity, the small intestine, or the HPV to assess the functional significance of the neuronal pathways identified by viral tracing.